This Can Travel Across Galaxies Alpha Beta or Gamma
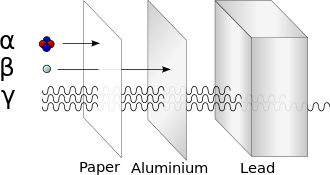
Analogy of the relative abilities of 3 unlike types of ionizing radiation to penetrate solid thing. Typical alpha particles (α) are stopped by a sheet of paper, while beta particles (β) are stopped by an aluminum plate. Gamma radiation (γ) is dampened when information technology penetrates lead. Notation caveats in the text virtually this simplified diagram.[ description needed ]

The international symbol for types and levels of ionizing radiation (radioactivity) that are unsafe for unshielded humans. Radiations, in general, exists throughout nature, such equally in light and sound.
In physics, radiations is the emission or transmission of energy in the form of waves or particles through infinite or through a material medium. [1] [2] This includes:
- electromagnetic radiation, such as radio waves, microwaves, infrared, visible light, ultraviolet, 10-rays, and gamma radiation (γ)
- particle radiations, such every bit alpha radiation (α), beta radiations (β), proton radiations and neutron radiations (particles of not-zip rest free energy)
- acoustic radiation, such as ultrasound, sound, and seismic waves (dependent on a physical transmission medium)
- gravitational radiation, that takes the form of gravitational waves, or ripples in the curvature of spacetime
Radiation is oft categorized as either ionizing or non-ionizing depending on the energy of the radiated particles. Ionizing radiation carries more ten eV, which is enough to ionize atoms and molecules and pause chemical bonds. This is an important distinction due to the large deviation in harmfulness to living organisms. A common source of ionizing radiations is radioactive materials that emit α, β, or γ radiation, consisting of helium nuclei, electrons or positrons, and photons, respectively. Other sources include X-rays from medical radiography examinations and muons, mesons, positrons, neutrons and other particles that constitute the secondary catholic rays that are produced after principal cosmic rays collaborate with World'due south atmosphere.
Gamma rays, X-rays and the higher energy range of ultraviolet low-cal constitute the ionizing role of the electromagnetic spectrum. The word "ionize" refers to the breaking of 1 or more electrons away from an atom, an activity that requires the relatively high energies that these electromagnetic waves supply. Farther down the spectrum, the non-ionizing lower energies of the lower ultraviolet spectrum cannot ionize atoms, merely tin can disrupt the inter-atomic bonds which grade molecules, thereby breaking down molecules rather than atoms; a good example of this is sunburn acquired by long-wavelength solar ultraviolet. The waves of longer wavelength than UV in visible lite, infrared and microwave frequencies cannot break bonds but tin can cause vibrations in the bonds which are sensed as oestrus. Radio wavelengths and below mostly are non regarded as harmful to biological systems. These are not abrupt delineations of the energies; there is some overlap in the furnishings of specific frequencies.[iii]
The discussion "radiations" arises from the phenomenon of waves radiating (i.e., traveling outward in all directions) from a source. This aspect leads to a system of measurements and physical units that are applicative to all types of radiations. Because such radiation expands as it passes through space, and as its energy is conserved (in vacuum), the intensity of all types of radiation from a betoken source follows an inverse-foursquare police in relation to the altitude from its source. Like whatsoever ideal police, the inverse-foursquare law approximates a measured radiation intensity to the extent that the source approximates a geometric point.
Ionizing radiation [edit]

Radiations with sufficiently high energy can ionize atoms; that is to say it tin knock electrons off atoms, creating ions. Ionization occurs when an electron is stripped (or "knocked out") from an electron trounce of the cantlet, which leaves the atom with a net positive charge. Because living cells and, more importantly, the DNA in those cells can exist damaged past this ionization, exposure to ionizing radiation is considered to increase the take chances of cancer. Thus "ionizing radiation" is somewhat artificially separated from particle radiation and electromagnetic radiations, but due to its great potential for biological damage. While an private prison cell is made of trillions of atoms, only a small fraction of those volition be ionized at low to moderate radiation powers. The probability of ionizing radiation causing cancer is dependent upon the absorbed dose of the radiation, and is a office of the damaging tendency of the blazon of radiations (equivalent dose) and the sensitivity of the irradiated organism or tissue (effective dose).
If the source of the ionizing radiation is a radioactive textile or a nuclear process such as fission or fusion, in that location is particle radiation to consider. Particle radiation is subatomic particles accelerated to relativistic speeds by nuclear reactions. Because of their momenta they are quite capable of knocking out electrons and ionizing materials, merely since virtually have an electrical charge, they don't take the penetrating power of ionizing radiations. The exception is neutron particles; see beneath. There are several different kinds of these particles, only the majority are blastoff particles, beta particles, neutrons, and protons. Roughly speaking, photons and particles with energies above about 10 electron volts (eV) are ionizing (some authorities apply 33 eV, the ionization free energy for water). Particle radiation from radioactive textile or cosmic rays well-nigh invariably carries enough free energy to be ionizing.
Most ionizing radiation originates from radioactive materials and space (cosmic rays), and as such is naturally present in the environment, since most rocks and soil accept small concentrations of radioactive materials. Since this radiation is invisible and not directly detectable by human senses, instruments such every bit Geiger counters are commonly required to detect its presence. In some cases, it may pb to secondary emission of visible light upon its interaction with matter, as in the case of Cherenkov radiation and radio-luminescence.
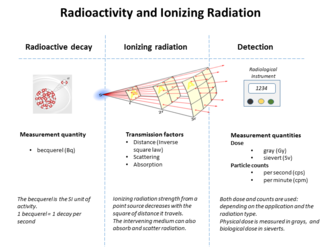
Graphic showing relationships between radioactivity and detected ionizing radiation
Ionizing radiations has many practical uses in medicine, research, and construction, but presents a health take a chance if used improperly. Exposure to radiation causes damage to living tissue; high doses result in Acute radiation syndrome (ARS), with peel burns, hair loss, internal organ failure, and decease, while any dose may result in an increased chance of cancer and genetic damage; a particular grade of cancer, thyroid cancer, often occurs when nuclear weapons and reactors are the radiations source because of the biological proclivities of the radioactive iodine fission product, iodine-131.[iv] However, calculating the exact run a risk and chance of cancer forming in cells caused by ionizing radiation is even so not well understood and currently estimates are loosely determined by population based data from the atomic bombings of Hiroshima and Nagasaki and from follow-up of reactor accidents, such as the Chernobyl disaster. The International Commission on Radiological Protection states that "The Commission is aware of uncertainties and lack of precision of the models and parameter values", "Collective effective dose is non intended every bit a tool for epidemiological adventure cess, and it is inappropriate to use information technology in risk projections" and "in item, the calculation of the number of cancer deaths based on collective effective doses from trivial private doses should exist avoided."[5]
Ultraviolet radiation [edit]
Ultraviolet, of wavelengths from 10 nm to 125 nm, ionizes air molecules, causing it to be strongly captivated by air and by ozone (O3) in item. Ionizing UV therefore does non penetrate Earth'south atmosphere to a significant degree, and is sometimes referred to every bit vacuum ultraviolet. Although present in space, this part of the UV spectrum is not of biological importance, because information technology does not attain living organisms on Earth.
There is a zone of the atmosphere in which ozone absorbs some 98% of non-ionizing merely unsafe UV-C and UV-B. This so-chosen ozone layer starts at about twenty miles (32 km) and extends upwards. Some of the ultraviolet spectrum that does achieve the basis is non-ionizing, but is even so biologically hazardous due to the ability of single photons of this energy to crusade electronic excitation in biological molecules, and thus damage them by means of unwanted reactions. An case is the formation of pyrimidine dimers in Dna, which begins at wavelengths below 365 nm (3.4 eV), which is well below ionization free energy. This property gives the ultraviolet spectrum some of the dangers of ionizing radiation in biological systems without actual ionization occurring. In contrast, visible lite and longer-wavelength electromagnetic radiation, such as infrared, microwaves, and radio waves, consists of photons with too niggling energy to cause damaging molecular excitation, and thus this radiations is far less chancy per unit of energy.
X-rays [edit]
X-rays are electromagnetic waves with a wavelength less than almost 10−9 m (greater than 3x1017 Hz and 1,240 eV). A smaller wavelength corresponds to a higher energy according to the equation Due east=h c/λ. ("E" is Free energy; "h" is Planck's constant; "c" is the speed of light; "λ" is wavelength.) When an X-ray photon collides with an cantlet, the atom may absorb the free energy of the photon and heave an electron to a higher orbital level or if the photon is extremely energetic, it may knock an electron from the atom altogether, causing the cantlet to ionize. Generally, larger atoms are more likely to blot an Ten-ray photon since they accept greater free energy differences between orbital electrons. The soft tissue in the human body is composed of smaller atoms than the calcium atoms that make up bone, so at that place is a contrast in the absorption of 10-rays. X-ray machines are specifically designed to take reward of the assimilation divergence betwixt bone and soft tissue, allowing physicians to examine construction in the man body.
X-rays are also totally absorbed by the thickness of the earth's temper, resulting in the prevention of the 10-ray output of the sun, smaller in quantity than that of UV but withal powerful, from reaching the surface.
Gamma radiation [edit]

Gamma (γ) radiation consists of photons with a wavelength less than 3x10−eleven meters (greater than x19 Hz and 41.four keV).[4] Gamma radiations emission is a nuclear process that occurs to rid an unstable nucleus of excess energy after about nuclear reactions. Both alpha and beta particles have an electric charge and mass, and thus are quite likely to interact with other atoms in their path. Gamma radiation, however, is composed of photons, which have neither mass nor electric charge and, equally a effect, penetrates much further through thing than either alpha or beta radiation.
Gamma rays can be stopped by a sufficiently thick or dumbo layer of material, where the stopping ability of the textile per given area depends mostly (but not entirely) on the total mass along the path of the radiations, regardless of whether the fabric is of loftier or low density. Yet, every bit is the case with X-rays, materials with a loftier atomic number such as lead or depleted uranium add a modest (typically 20% to xxx%) corporeality of stopping power over an equal mass of less dumbo and lower atomic weight materials (such as water or concrete). The temper absorbs all gamma rays approaching Earth from space. Even air is capable of absorbing gamma rays, halving the free energy of such waves past passing through, on the boilerplate, 500 ft (150 m).
Alpha radiation [edit]

Alpha particles are helium-4 nuclei (ii protons and two neutrons). They collaborate with matter strongly due to their charges and combined mass, and at their usual velocities only penetrate a few centimeters of air, or a few millimeters of low density material (such as the sparse mica textile which is specially placed in some Geiger counter tubes to let alpha particles in). This means that alpha particles from ordinary alpha disuse exercise not penetrate the outer layers of expressionless pare cells and cause no damage to the alive tissues below. Some very high energy alpha particles compose about 10% of cosmic rays, and these are capable of penetrating the trunk and even sparse metal plates. Still, they are of danger but to astronauts, since they are deflected by the Globe's magnetic field and and then stopped by its atmosphere.
Alpha radiations is dangerous when alpha-emitting radioisotopes are ingested or inhaled (breathed or swallowed). This brings the radioisotope shut enough to sensitive alive tissue for the alpha radiation to impairment cells. Per unit of measurement of energy, alpha particles are at to the lowest degree xx times more than effective at cell-damage as gamma rays and X-rays. See relative biological effectiveness for a give-and-take of this. Examples of highly poisonous alpha-emitters are all isotopes of radium, radon, and polonium, due to the amount of decay that occur in these brusk one-half-life materials.
Beta radiations [edit]

Beta-minus (β−) radiations consists of an energetic electron. Information technology is more penetrating than alpha radiations but less than gamma. Beta radiation from radioactive decay can be stopped with a few centimeters of plastic or a few millimeters of metallic. It occurs when a neutron decays into a proton in a nucleus, releasing the beta particle and an antineutrino. Beta radiation from linac accelerators is far more energetic and penetrating than natural beta radiation. Information technology is sometimes used therapeutically in radiotherapy to treat superficial tumors.
Beta-plus (β+) radiation is the emission of positrons, which are the antimatter form of electrons. When a positron slows to speeds similar to those of electrons in the material, the positron will annihilate an electron, releasing 2 gamma photons of 511 keV in the process. Those two gamma photons volition be traveling in (approximately) contrary direction. The gamma radiations from positron annihilation consists of high energy photons, and is besides ionizing.
Neutron radiations [edit]
Neutrons are categorized according to their speed/energy. Neutron radiation consists of costless neutrons. These neutrons may be emitted during either spontaneous or induced nuclear fission. Neutrons are rare radiation particles; they are produced in large numbers merely where concatenation reaction fission or fusion reactions are active; this happens for about x microseconds in a thermonuclear explosion, or continuously inside an operating nuclear reactor; production of the neutrons stops almost immediately in the reactor when it goes not-critical.
Neutrons can make other objects, or material, radioactive. This process, called neutron activation, is the primary method used to produce radioactive sources for apply in medical, academic, and industrial applications. Even comparatively low speed thermal neutrons cause neutron activation (in fact, they cause it more efficiently). Neutrons exercise non ionize atoms in the same way that charged particles such equally protons and electrons do (by the excitation of an electron), because neutrons have no charge. It is through their absorption past nuclei which then become unstable that they cause ionization. Hence, neutrons are said to be "indirectly ionizing." Fifty-fifty neutrons without significant kinetic energy are indirectly ionizing, and are thus a significant radiation hazard. Not all materials are capable of neutron activation; in water, for example, the most common isotopes of both types atoms present (hydrogen and oxygen) capture neutrons and get heavier but remain stable forms of those atoms. Simply the absorption of more than than ane neutron, a statistically rare occurrence, tin actuate a hydrogen atom, while oxygen requires two additional absorptions. Thus water is simply very weakly capable of activation. The sodium in table salt (equally in body of water water), on the other hand, need merely absorb a single neutron to become Na-24, a very intense source of beta decay, with half-life of 15 hours.
In addition, high-energy (high-speed) neutrons have the ability to directly ionize atoms. One mechanism by which high energy neutrons ionize atoms is to strike the nucleus of an atom and knock the atom out of a molecule, leaving ane or more than electrons behind as the chemical bond is broken. This leads to production of chemical free radicals. In improver, very high energy neutrons can crusade ionizing radiation by "neutron spallation" or knockout, wherein neutrons cause emission of high-energy protons from atomic nuclei (especially hydrogen nuclei) on impact. The last process imparts virtually of the neutron'due south energy to the proton, much like one billiard ball hitting some other. The charged protons and other products from such reactions are directly ionizing.
High-free energy neutrons are very penetrating and can travel great distances in air (hundreds or even thousands of meters) and moderate distances (several meters) in common solids. They typically require hydrogen rich shielding, such as concrete or water, to cake them within distances of less than a meter. A common source of neutron radiation occurs inside a nuclear reactor, where a meters-thick water layer is used as effective shielding.
Cosmic radiation [edit]
There are two sources of loftier energy particles entering the Earth's atmosphere from outer space: the sun and deep infinite. The sun continuously emits particles, primarily free protons, in the solar current of air, and occasionally augments the flow hugely with coronal mass ejections (CME).
The particles from deep space (inter- and extra-galactic) are much less frequent, simply of much higher energies. These particles are likewise mostly protons, with much of the remainder consisting of helions (alpha particles). A few completely ionized nuclei of heavier elements are present. The origin of these galactic cosmic rays is not yet well understood, but they seem to be remnants of supernovae and peculiarly gamma-ray bursts (GRB), which feature magnetic fields capable of the huge accelerations measured from these particles. They may also exist generated by quasars, which are galaxy-wide jet phenomena similar to GRBs only known for their much larger size, and which seem to be a tearing office of the universe's early history.
Non-ionizing radiations [edit]

The kinetic energy of particles of not-ionizing radiation is likewise small to produce charged ions when passing through matter. For not-ionizing electromagnetic radiation (see types below), the associated particles (photons) have merely sufficient energy to alter the rotational, vibrational or electronic valence configurations of molecules and atoms. The effect of not-ionizing forms of radiations on living tissue has only recently been studied. Nevertheless, unlike biological furnishings are observed for unlike types of non-ionizing radiations.[4] [6]
Even "non-ionizing" radiation is capable of causing thermal-ionization if it deposits plenty heat to raise temperatures to ionization energies. These reactions occur at far higher energies than with ionization radiation, which requires only single particles to cause ionization. A familiar example of thermal ionization is the flame-ionization of a common burn down, and the browning reactions in common food items induced by infrared radiation, during broiling-type cooking.
The electromagnetic spectrum is the range of all possible electromagnetic radiations frequencies.[4] The electromagnetic spectrum (usually just spectrum) of an object is the characteristic distribution of electromagnetic radiation emitted past, or absorbed past, that particular object.
The non-ionizing portion of electromagnetic radiations consists of electromagnetic waves that (as individual quanta or particles, run into photon) are not energetic enough to disassemble electrons from atoms or molecules and hence cause their ionization. These include radio waves, microwaves, infrared, and (sometimes) visible light. The lower frequencies of ultraviolet lite may cause chemic changes and molecular damage similar to ionization, but is technically not ionizing. The highest frequencies of ultraviolet light, every bit well as all Ten-rays and gamma-rays are ionizing.
The occurrence of ionization depends on the energy of the individual particles or waves, and not on their number. An intense overflowing of particles or waves will non cause ionization if these particles or waves practise not carry enough energy to be ionizing, unless they enhance the temperature of a body to a indicate high enough to ionize minor fractions of atoms or molecules by the process of thermal-ionization (this, withal, requires relatively extreme radiations intensities).
Ultraviolet light [edit]
As noted above, the lower part of the spectrum of ultraviolet, chosen soft UV, from 3 eV to most 10 eV, is non-ionizing. However, the effects of non-ionizing ultraviolet on chemistry and the damage to biological systems exposed to it (including oxidation, mutation, and cancer) are such that even this part of ultraviolet is often compared with ionizing radiation.
Visible lite [edit]
Light, or visible light, is a very narrow range of electromagnetic radiation of a wavelength that is visible to the man heart, or 380–750 nm which equates to a frequency range of 790 to 400 THz respectively.[iv] More than broadly, physicists use the term "light" to mean electromagnetic radiation of all wavelengths, whether visible or not.
Infrared [edit]
Infrared (IR) lite is electromagnetic radiations with a wavelength between 0.7 and 300 micrometers, which corresponds to a frequency range between 430 and 1 THz respectively. IR wavelengths are longer than that of visible light, but shorter than that of microwaves. Infrared may exist detected at a distance from the radiating objects by "feel." Infrared sensing snakes can detect and focus infrared by employ of a pinhole lens in their heads, called "pits". Bright sunlight provides an irradiance of merely over 1 kilowatt per square meter at sea level. Of this energy, 53% is infrared radiation, 44% is visible light, and iii% is ultraviolet radiations.[four]
Microwave [edit]
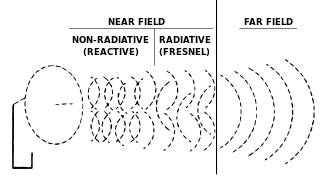
In electromagnetic radiation (such equally microwaves from an antenna, shown here) the term "radiation" applies simply to the parts of the electromagnetic field that radiate into infinite space and decrease in intensity past an inverse-foursquare law of power and so that the total radiations energy that crosses through an imaginary spherical surface is the same, no matter how far abroad from the antenna the spherical surface is drawn. Electromagnetic radiation includes the far field part of the electromagnetic field effectually a transmitter. A role of the "virtually-field" close to the transmitter, is part of the changing electromagnetic field, but does not count as electromagnetic radiation.
Microwaves are electromagnetic waves with wavelengths ranging from as short as 1 millimeter to as long every bit one meter, which equates to a frequency range of 300 MHz to 300 GHz. This wide definition includes both UHF and EHF (millimeter waves), but various sources use dissimilar other limits.[iv] In all cases, microwaves include the entire super high frequency band (3 to thirty GHz, or 10 to i cm) at minimum, with RF engineering often putting the lower boundary at 1 GHz (xxx cm), and the upper around 100 GHz (3mm).
Radio waves [edit]
Radio waves are a type of electromagnetic radiation with wavelengths in the electromagnetic spectrum longer than infrared light. Like all other electromagnetic waves, they travel at the speed of light. Naturally occurring radio waves are made past lightning, or by certain astronomical objects. Artificially generated radio waves are used for fixed and mobile radio communication, broadcasting, radar and other navigation systems, satellite communication, figurer networks and innumerable other applications. In addition, nearly any wire carrying alternating current will radiate some of the energy away as radio waves; these are generally termed interference. Unlike frequencies of radio waves accept dissimilar propagation characteristics in the Earth's atmosphere; long waves may bend at the rate of the curvature of the Earth and may cover a function of the Earth very consistently, shorter waves travel effectually the earth past multiple reflections off the ionosphere and the Earth. Much shorter wavelengths bend or reflect very little and travel along the line of sight.
Very depression frequency [edit]
Very depression frequency (VLF) refers to a frequency range of 30 Hz to 3 kHz which corresponds to wavelengths of 100,000 to 10,000 meters respectively. Since at that place is not much bandwidth in this range of the radio spectrum, only the very simplest signals can exist transmitted, such equally for radio navigation. Also known as the myriameter band or myriameter moving ridge as the wavelengths range from ten to ane myriameter (an obsolete metric unit equal to 10 kilometers).
Extremely low frequency [edit]
Extremely low frequency (ELF) is radiation frequencies from 3 to thirty Hz (108 to 107 meters respectively). In atmosphere science, an alternative definition is ordinarily given, from 3 Hz to 3 kHz.[iv] In the related magnetosphere science, the lower frequency electromagnetic oscillations (pulsations occurring below ~3 Hz) are considered to lie in the ULF range, which is thus also defined differently from the ITU Radio Bands. A massive military ELF antenna in Michigan radiates very slow letters to otherwise unreachable receivers, such as submerged submarines.
Thermal radiation (heat) [edit]
Thermal radiation is a common synonym for infrared radiation emitted by objects at temperatures often encountered on World. Thermal radiation refers not only to the radiation itself, but also the procedure by which the surface of an object radiates its thermal energy in the form of black body radiations. Infrared or red radiation from a common household radiator or electric heater is an example of thermal radiations, as is the rut emitted by an operating incandescent calorie-free bulb. Thermal radiations is generated when energy from the move of charged particles within atoms is converted to electromagnetic radiations.
As noted higher up, even low-frequency thermal radiation may cause temperature-ionization whenever information technology deposits sufficient thermal energy to raise temperatures to a loftier enough level. Common examples of this are the ionization (plasma) seen in mutual flames, and the molecular changes acquired by the "browning" during nutrient-cooking, which is a chemic process that begins with a large component of ionization.
Black-torso radiation [edit]
Black-body radiation is an idealized spectrum of radiation emitted by a trunk that is at a uniform temperature. The shape of the spectrum and the total amount of free energy emitted by the body is a function of the absolute temperature of that body. The radiation emitted covers the entire electromagnetic spectrum and the intensity of the radiation (power/unit-area) at a given frequency is described by Planck'due south constabulary of radiation. For a given temperature of a blackness-body in that location is a detail frequency at which the radiation emitted is at its maximum intensity. That maximum radiations frequency moves toward higher frequencies as the temperature of the body increases. The frequency at which the blackness-body radiations is at maximum is given by Wien's displacement police force and is a office of the trunk'due south accented temperature. A blackness-trunk is one that emits at any temperature the maximum possible amount of radiation at any given wavelength. A black-body will also absorb the maximum possible incident radiation at whatever given wavelength. A blackness-torso with a temperature at or below room temperature would thus appear absolutely black, every bit it would not reflect any incident low-cal nor would information technology emit enough radiations at visible wavelengths for our eyes to notice. Theoretically, a blackness-body emits electromagnetic radiation over the entire spectrum from very low frequency radio waves to x-rays, creating a continuum of radiation.
The color of a radiating black-body tells the temperature of its radiating surface. It is responsible for the colour of stars, which vary from infrared through carmine (ii,500K), to yellow (v,800K), to white and to blue-white (15,000K) as the peak radiance passes through those points in the visible spectrum. When the peak is below the visible spectrum the body is black, while when it is above the body is blue-white, since all the visible colors are represented from blue decreasing to cherry-red.
Discovery [edit]
Electromagnetic radiation of wavelengths other than visible lite were discovered in the early 19th century. The discovery of infrared radiation is ascribed to William Herschel, the astronomer. Herschel published his results in 1800 earlier the Imperial Society of London. Herschel, like Ritter, used a prism to refract light from the Sun and detected the infrared (beyond the ruby function of the spectrum), through an increment in the temperature recorded past a thermometer.
In 1801, the German physicist Johann Wilhelm Ritter fabricated the discovery of ultraviolet by noting that the rays from a prism darkened argent chloride preparations more chop-chop than violet lite. Ritter's experiments were an early forerunner to what would become photography. Ritter noted that the UV rays were capable of causing chemical reactions.
The first radio waves detected were not from a natural source, simply were produced deliberately and artificially by the German scientist Heinrich Hertz in 1887, using electrical circuits calculated to produce oscillations in the radio frequency range, following formulas suggested by the equations of James Clerk Maxwell.
Wilhelm Röntgen discovered and named X-rays. While experimenting with high voltages practical to an evacuated tube on eight Nov 1895, he noticed a fluorescence on a nearby plate of coated glass. Inside a month, he discovered the main properties of 10-rays that nosotros understand to this 24-hour interval.
In 1896, Henri Becquerel found that rays emanating from certain minerals penetrated blackness paper and acquired fogging of an unexposed photographic plate. His doctoral student Marie Curie discovered that simply certain chemic elements gave off these rays of free energy. She named this beliefs radioactivity.
Alpha rays (alpha particles) and beta rays (beta particles) were differentiated by Ernest Rutherford through simple experimentation in 1899. Rutherford used a generic pitchblende radioactive source and determined that the rays produced past the source had differing penetrations in materials. I type had brusk penetration (it was stopped by paper) and a positive charge, which Rutherford named alpha rays. The other was more penetrating (able to expose flick through newspaper but not metal) and had a negative charge, and this type Rutherford named beta. This was the radiation that had been first detected by Becquerel from uranium salts. In 1900, the French scientist Paul Villard discovered a third neutrally charged and particularly penetrating blazon of radiation from radium, and afterwards he described it, Rutherford realized it must exist yet a third blazon of radiation, which in 1903 Rutherford named gamma rays.
Henri Becquerel himself proved that beta rays are fast electrons, while Rutherford and Thomas Royds proved in 1909 that blastoff particles are ionized helium. Rutherford and Edward Andrade proved in 1914 that gamma rays are like X-rays, only with shorter wavelengths.
Cosmic ray radiations striking the Earth from outer space were finally definitively recognized and proven to be in 1912, every bit the scientist Victor Hess carried an electrometer to various altitudes in a free balloon flight. The nature of these radiations was simply gradually understood in later years.
The Neutron and neutron radiation were discovered by James Chadwick in 1932. A number of other loftier energy particulate radiations such as positrons, muons, and pions were discovered by cloud bedroom examination of catholic ray reactions shortly thereafter, and others types of particle radiation were produced artificially in particle accelerators, through the final half of the twentieth century.
Applications [edit]
Medicine [edit]
Radiations and radioactive substances are used for diagnosis, treatment, and research. 10-rays, for example, pass through muscles and other soft tissue merely are stopped by dense materials. This belongings of 10-rays enables doctors to find broken basic and to locate cancers that might be growing in the torso.[7] Doctors also find certain diseases by injecting a radioactive substance and monitoring the radiation given off as the substance moves through the body.[8] Radiation used for cancer treatment is called ionizing radiation considering it forms ions in the cells of the tissues it passes through as it dislodges electrons from atoms. This can kill cells or change genes so the cells cannot abound. Other forms of radiation such as radio waves, microwaves, and lite waves are called not-ionizing. They don't accept equally much energy then they are not able to ionize cells.[9]
Advice [edit]
All modern communication systems utilise forms of electromagnetic radiations. Variations in the intensity of the radiations represent changes in the sound, pictures, or other information being transmitted. For instance, a human voice can be sent equally a radio wave or microwave past making the wave vary to corresponding variations in the voice. Musicians accept also experimented with gamma rays sonification, or using nuclear radiation, to produce sound and music.[10]
Science [edit]
Researchers apply radioactive atoms to determine the age of materials that were once part of a living organism. The historic period of such materials tin can be estimated past measuring the amount of radioactive carbon they contain in a process called radiocarbon dating. Similarly, using other radioactive elements, the age of rocks and other geological features (even some man-made objects) can exist determined; this is chosen Radiometric dating. Environmental scientists apply radioactive atoms, known every bit tracer atoms, to identify the pathways taken by pollutants through the surround.
Radiation is used to determine the limerick of materials in a process chosen neutron activation analysis. In this procedure, scientists bombard a sample of a substance with particles called neutrons. Some of the atoms in the sample absorb neutrons and get radioactive. The scientists can identify the elements in the sample past studying the emitted radiations.
Possible harm to health and environment from certain types of radiation [edit]
Ionizing radiation in certain weather condition can crusade damage to living organisms, causing cancer or genetic damage.[4]
Not-ionizing radiations in certain conditions likewise can cause damage to living organisms, such as burns. In 2011, the International Agency for Inquiry on Cancer (IARC) of the Globe Health Organization (WHO) released a statement adding radio frequency electromagnetic fields (including microwave and millimeter waves) to their listing of things which are possibly carcinogenic to humans.[xi]
RWTH Aachen University's EMF-Portal web site presents ane of the biggest database about the effects of Electromagnetic radiation. As of 12 July 2022 it has 28,547 publications and 6,369 summaries of private scientific studies on the effects of electromagnetic fields.[12]
Run into also [edit]
- Australian Radiation Protection and Nuclear Rubber Agency (ARPANSA)
- Background radiation, which really refers to background ionizing radiation
- Banana equivalent dose
- Cherenkov radiations
- Cosmic microwave background radiation, 3 1000 blackbody radiation that fills the Universe
- Electromagnetic spectrum
- Hawking radiation
- Ionizing radiations
- Non-ionizing radiation
- Radiant energy, radiation by a source into the surrounding environment.
- Radiations impairment – agin effects of ionizing radiation on materials and devices
- Radiation hardening – making electronics resistant to failure in high ionizing radiation environments
- Radiation hormesis – ionizing radiation dosage threshold damage theory
- Radiation poisoning – adverse furnishings of ionizing radiation on life forms
- Radiation properties
- Radiations Protection Convention, 1960 – by International Labour Organization
- Radioactive contamination
- Radioactive decay
Notes and references [edit]
- ^ Weisstein, Eric Due west. "Radiations". Eric Weisstein's World of Physics. Wolfram Enquiry. Retrieved 11 Jan 2014.
- ^ "Radiation". The costless dictionary by Farlex. Farlex, Inc. Retrieved 11 January 2014.
- ^ "The Electromagnetic Spectrum". Centers for Affliction Control and Prevention. seven December 2015. Retrieved 29 Baronial 2018.
- ^ a b c d east f grand h i Kwan-Hoong Ng (xx–22 October 2003). "Non-Ionizing Radiations – Sources, Biological Effects, Emissions and Exposures" (PDF). Proceedings of the International Conference on Non-Ionizing Radiation at UNITEN ICNIR2003 Electromagnetic Fields and Our Health.
- ^ "ICRP Publication 103 The 2007 Recommendations of the International Commission on Protection" (PDF). ICRP. Retrieved 12 Dec 2013.
- ^ Moulder, John E. "Static Electric and Magnetic Fields and Human Health". Archived from the original on 14 July 2007.
- ^ Radiography
- ^ Nuclear medicine
- ^ Bellenir, Karen (2007). Cancer Sourcebook. Detroit, MI: Omnigraphics. pp. 112–113. ISBN978-0-7808-0947-5.
- ^ Dunn, Peter (2014). "Making Nuclear Music". Slice of MIT. Retrieved 29 Baronial 2018.
- ^ "IARC Classifies Radiofrequency Electromagnetic Fields Equally Possibly Carcinogenic To Humans" (PDF) (Press release). The WHO/International Bureau for Research on Cancer (IARC). 31 May 2011.
- ^ "EMF-Portal". Retrieved 12 July 2019.
External links [edit]
- Radiations on In Our Time at the BBC
- Health Physics Society Public Instruction Website
- Ionizing Radiation and Radon from World Wellness System
- Q&A: Health effects of radiations exposure, BBC News, 21 July 2011.
- John Tyndall (1865), On Radiations: the "Rede" Lecture delivered in the Senate-House before the University of Cambridge on Tuseday, May 16, 1865, Rede Lecture (1st ed.), London: Longman, LCCN 05005356, OCLC 4920745, Wikidata Q19086230
Source: https://en.wikipedia.org/wiki/Radiation
0 Response to "This Can Travel Across Galaxies Alpha Beta or Gamma"
إرسال تعليق